Scientific Consulting & Solar Generators
Specializing in Silicon Photovoltaics
| home |
| experience |
| solar generators |
| vision systems |
| publications |
| patents |
| behind the name |
| contact |
|
The Story of Harvesting Lightdrops |
|
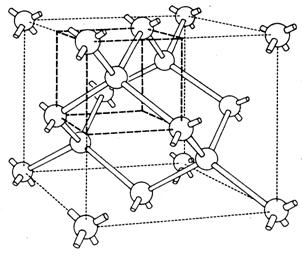
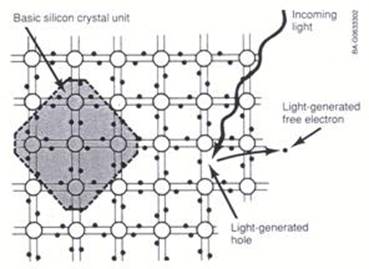
The solar cells made from crystalline silicon are almost magical devices. They take sunlight shining on them and convert it into electricity. Electricity is a flow of electrons, but where do these electrons come from, and how do these thin sheets of processed silicon manage to accomplish this feat? The electrons are part of the silicon material. As shown in Figure 1, each silicon atom is connected to four neighboring silicon atoms by means of its four outermost electrons. Each bond between two silicon atoms is made of two electrons, one contributed by the first atom and the other contributed by the second atom. These electrons are locked into a bound state, and as such are not able to participate in the flow of electric current. They must first be freed. This is where light comes in. Just as rainfall is made up of a stream of individual raindrops, "lightfall" (sunshine) is made up of a stream of individual "lightdrops" called photons. A photon is a tiny packet of energy, too small to be sensed individually by our eyes. But each photon in the visible part of the spectrum packs enough of a punch to knock a single electron free from its bound state. As a free particle, the electron is now capable of joining with other electrons as part of a flow of electric current. Thus, the stream of photons that we know as sunlight penetrates into the silicon where each photon surrenders its energy to free a bound electron, and "dies" (is absorbed) in the process. This is the nature of the interaction of light with silicon (and with other semiconductors), and is illustrated in Figure 2.
Figure 1. Silicon atoms bound together by electrons.
Freeing the electrons is only part of the job. We must also collect or “harvest” these freed electrons by getting them out of the silicon and into an external circuit to do the work of electricity, such as spinning the impeller of a pump. Electrons do not stay free in the silicon for very long, only about 1 millisecond (0.001 second), before they fall back into a hole left by some other freed electron and become bound again. To get the electron out, we exploit the fact that the electron is negatively charged. Strong electric fields are built into the solar cell near its front side by a phosphorus diffusion, and near its rear side by adding boron or aluminum alloying. These electric fields propel freed electrons toward the front surface of the cell where they leave the silicon and ride the silver grid lines to the external circuit. After traveling along the wires of the external circuit, electrons return from their journey and pass into the aluminum on the rear side of the cell and then fall into holes in the nearby silicon which were created when other electrons were freed. Thus, the action of the sunlight is to free electrons from their bound state, and the action of the built-in electric fields is to pump these electrons around the external circuit. To get more light into the silicon (and to give the cell a pleasing blue color), an anti‑reflective coating is often deposited on the front of the cell.
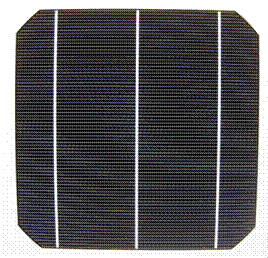
Figure 2. Action of photons in freeing bound electrons State-of-the-art crystalline silicon solar cells generate over 0.5 Volt when illuminated under standard test conditions, equivalent to bright noonday sun, while the current produced depends on cell area. Standard production cells, 156 mm pseudosquare, produce over 8 Amps of current. This translates to over 4 Watts of power generated per cell. Such a cell, shown in Figure 3, thus “harvests” the electrons produced by absorbing the “lightdrops” of sunshine.
Figure 3. Typical crystalline silicon solar cell.
|